Solids separation in manure handling systems
Author:
Douglas W. Hamilton, Oklahoma State University
Reviewer:
John Classen, North Carolina State University;
Robert Burns, Iowa State University
Introduction
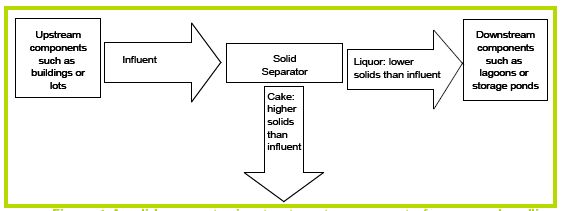
A solids separator removes solids from mixtures of solids and liquids. It is appropriate to think of a solids separator as a device that breaks an incoming waste stream into two separate flows: one with a solids content lower than the original waste stream, the other with a solids content higher than the original waste stream (Figure 1). Depending on the type of separator used, the lower-solids content waste stream may be called effluent, separated liquids, or liquor. Likewise, the higher-solids stream might be called sludge, grit, separated solids, or cake. Individual manufacturers may use different terms to describe the same stream. To keep things simple, in this paper we will call the stream entering a solids separator influent, the low solids stream leaving the separator liquor, and the high solids stream cake. Devices remove solids by a number of methods: settling material to the bottom of a waste stream, screening larger particles out of the stream, and floating light material to the top of the stream, to name a few. Some devices may perform more than one type of removal and create three or more waste streams, the idea of a solids separator is to create at least one stream of low-solids liquor.
Objectives
Introduce concepts of solids separation
Describe methods used for the separation of solids including:
- Settling
- Screening
- Filtering
- Centrifugal force
- Flotation
- Chemical enhancement
Mass and volume relationships in solids separation
Volume, mass, and flow. Solids separators work on streams flowing through manure handling systems. That makes it easiest to describe the devices based on the rate at which material flows through them. Flow rate can be either volume or mass per time.
A solids separator divides the incoming stream into two or more outgoing streams, and no material is lost in the process. Flow in must equal flow out. Mathematically, this is written as:
(1) Qi = Qc + Ql Where Qi is influent flow, Qc is the flow of cake, and Ql is liquor flow.
The mass or amount of material flowing into a separator must also equal the mass leaving the separator. Summing mass flow through a device gives the mathematical equation:
(2) Mi = Mc + Ml
Where Mi is the influent mass flow rate, Mc is the cake mass flow rate, and Ml is the liquor mass flow rate. The material represented by M can be anything. It can be the mass flow rate of solids, the mass flow rate of a mixture of solids and liquids, the mass flow rate of nitrogen, or whatever you choose.
Sometimes, we may want to know the total volume or total mass of material separated from the waste stream over a certain period of time. It is easy to move from volumetric (liquid) flow rate to volume, and from mass flow rate to mass. Just remember, volumetric flow rate multiplied by time equals volume, and mass flow rate multiplied by time equals mass:
(3) V = Qt
(4) m = Mt
Where is V is volume moved during a certain time, t, and m is mass moved during the time t. You can also move from volume to volumetric flow rate and from mass to mass flow rate, by collecting a certain amount of material from the stream and dividing it by the time needed to collect the material:
(5) Q = V/t
(6) M = m/t
Concentration ties mass flow rate to volumetric flow rate. A simple relationship ties mass and volumetric flow rates together. The mass of material in a given volume is equal to its concentration times the volume. In terms of flow, the mass flow rate of any material in a stream is the concentration of that material times the liquid flow rate:
(7) M = QC
Where C is concentration. In this case, C has units of mass per volume.
Combining Equations 1 and 7 gives the following relationship that defines mass and volumetric flows through a solids separator:
(8) QiCi = QcCc + QlCl
Where Ci is concentration of influent, Cc is concentration of cake and Cl is concentration of liquor.
Things can get confusing because we often use mass fraction (mass of a particular element divided by the mass of the total flow) to describe concentration. In this case, Equation 7 becomes:
(9) Ma = MXa
Where Ma is the mass flow rate of material a, M is the mass flow rate of the total waste stream, and Xa is mass fraction of the material a in the waste stream. When mass fraction is given in percent by weight, Equation 9 becomes:
(10) Ma = MXa/100
Measuring solids separation efficiency and effectiveness
There are two ways to measure how efficiently a solids separator operates: separation efficiency and mass removal efficiency.
Separation efficiency
Manufacturers often state the efficiency of their equipment using the difference between influent and liquor solids concentration. This can be defined as fraction of solids remaining in the liquor, or more accurately, fraction passing through the separator:
(11) Fraction Passing = Cl/Ci
Turning this around, we could define efficiency by the fraction removed from the waste stream. This is the separation efficiency:
(12) Separation Efficiency = Cc/Ci = (Ci-Cl)/Ci
If you do the algebra, separation efficiency and fraction passing are related by:
(13) (Ci-Cl)/Ci = 1- Cl/Ci
Or in words: separation efficiency equals one minus fraction passing.
Separation efficiency and fraction passing show how well the separator improves the quality of liquor flowing to downstream components. They are useful when evaluating the potential for a treatment scheme to change the waste. Change in solids concentration does not tell you the mass of solids removed from the waste stream, though.
Mass removal efficiency
Most of the time, we are interested in the mass of solids removed from the waste stream and transferred to cake. Mass removal efficiency is defined as:
(14) Mass Removal Efficiency = Mc/Mi = (Mi-Ml)/Mi
Using the relationships between mass flow rate, liquid flow rate, and concentration, we can also write this as:
(15) Mass Removal Efficiency = (CiQi – ClQl)/CiQi
If influent flow rate were equal to liquor flow rate, mass removal efficiency would be the same as separation efficiency, but Qi does not equal Ql since some liquid always leaves with cake. Mass removal efficiency and separation efficiency are not the same. They can be close when the volume of cake removed is small, but they are never exactly the same.
We need make three measurements to solve for mass removal efficiency. Three out of a possible six: Qi, Qc, Ql, Ci ,Cc, and Cl .
Measures of separator effectiveness
We might want to include Cc in the measures we make to determine efficiency. In the next section, we will see how the physical behavior of mixtures depends on solids concentration. The concentration of solids determines whether you pump, scoop, scrape, or push cake away from the separator.
Also according to Equation 8, we need to know at least two of the three concentrations in order to determine flow rates downstream of the separator. Not knowing the downstream flow rates before we install a solids separator can be disastrous!
Consistency and dryness of manure
Consistency
Defines the physical properties of manure. Unfrozen manure can exist in four different physical states:
Liquid: The mixture always acts like a liquid (pours, spreads out) and does not contain suspended solid material.
Slurry: The mixture acts like a liquid, and contains identifiable suspended solids.
Semi-Solid: The mixture generally acts like a solid (does not pour, is stackable), but when put under pressure can flow like a liquid (fresh cow patties squirt when stepped on).
Solid: The mixture always acts like a solid.

Two factors determine consistency: solids content and shape of the suspended solids. Figure 2 shows how consistency of manure of various animals depends on solids content.
The dotted line in Figure 2 is the solids content at which manure is excreted by animals. Swine manure is usually excreted as slurry. You must use caution with Figure 2, however. The dotted line only shows consistency as manure is excreted. Add water, bedding, or let the excreted manure dry, and consistency changes.
The consistency of a liquid may not tell anything about the “dryness” of material. Piles of solids and semisolids may ooze liquids even though the material stacks like a solid. Dryness is determined by how tightly liquids are held by solids. Gravitational liquids are loosely bound, and can move by gravity. Gravitational water can easily move through a stack of solids and seep out of the stack. Hygroscopic liquids are adsorbed onto solid surfaces. Gravity will not move hygroscopic water, but the solids may feel wet. The amount of hygroscopic water held by solids depends on the temperature and humidity of the surrounding atmosphere. Absorbed liquids are liquids that have become part of the solids themselves. Absorbed liquid can be transferred to hygroscopic or gravitational liquids by compressing, crushing, or drying the material.
Solids and moisture content
The most basic definition of solids content is, “solids are what is left after all the liquid has been evaporated from a mixture of solids and liquids”. We call the solids left after evaporation Total Solids, or TS for short. Total Solids are defined as the mass of solids remaining after a sample has been placed in a 103oC oven for one day, divided by the original mass of the sample. Total solids of thick slurries are reported on a percent basis. For dilute slurries, TS may be expressed as mass over volume, most commonly mg/l.
As the TS concentration increases, and slurries start acting as semi-solids, the method of expressing solid/liquid relationship of mixtures switches from solids content to moisture content. Moisture content is defined as the mass of liquid in semisolid material divided by the wet mass of the material. As long as you stick to a wet weight basis for determining solids and moisture content, the two values are additive:
(16) Moisture Content (%) + Solids Content (%) = 100
Moisture content of very dry materials, such as soil, are sometimes measured as the mass of liquid divided by mass of dried material, but this definition is hardly ever used with manure.
Solids fractions
Total solids only tell how many solids are in a liquid or slurry. It does not say much about what kind of solids are present. Total solids can be broken into four fractions based on two distinctions:
- Is the solid suspended by or dissolved in the liquid?
- Is the solid made of organic or inorganic material?
A particle is considered dissolved if it can pass through a filter with holes 1.5 microns in diameter (1/17,000 inch). If the 1.5 micron filter stops a particle, it is considered to be suspended. The 1.5 micron limit means that most bacteria are considered suspended, while viruses are dissolved. Silt particles, which are 2 microns and larger, are suspended. Most clay particles, although technically suspended in the liquid, are by definition, dissolved, since they are smaller than 2 microns. The distinction between organic and inorganic is determined by whether or not the solid will burn if placed in a 550oC furnace.
Using these two tests, Total solids can be divided into two four fractions:
(17) TS = VSS + FSS + VDS + FDS
Where: VSS=Volatile Suspended Solids, organic particles larger than 1.5 microns
FSS=Fixed Suspended Solids, inorganic particles larger than 1.5 microns
VDS=Volatile Dissolved Solids, dissolved organic chemicals and some organic particles smaller than 1.5 microns.
FDS=Fixed Dissolved Solids, dissolved inorganic chemicals (salt content), clay and mineral particles smaller than 1.5 microns.
Grouping the four fractions according to these characteristics gives four more measures of solids content:
(18) TVS = Total Volatile Solids = VSS + VDS
(19) TFS = Total Fixed Solids = FSS + FDS
(20) TSS = Total Suspended Solids = VSS + FSS
(21) TDS = Total Dissolved Solids = VDS + FDS
Other important measurements
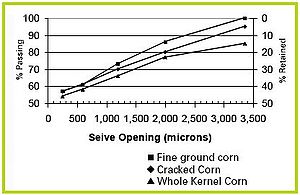
Particle size distribution
Very rarely are all the suspended solids the same size. Usually, particles are an assortment, or distribution, of different sizes. To determine particle size distribution, a wet manure sample is passed through a series of sieves with increasingly smaller openings. Distribution is measured as the weight percentage of TS passing through each opening size, plotted on a graph such as the one shown in Figure 3.
Particle size distribution is very important in solids separation. Let’s say we passed the three distributions shown in figure 3 through a screen with 2,000 micron openings. We would expect 75% or more of the manure solids from pigs fed a diet of whole kernel corn to pass through the screen. More than 85% of the manure solids from pigs fed a diet of fine ground corn would pass through the screen. Knowing that separation efficiency is equal to one minus fraction passing, we would a expect 25% separation efficiency with a 2,000 micron screen if the pigs were fed a diet of whole kernel corn. Likewise, a 2,000 micron screen would have at best 15% separation efficiency if the pigs were fed with diet of fine ground corn.
Particle shape
The shape, as well as the size, of a particle determines if it passes through a screen. If all manure solids were balls, we would expect them to pass through a screen with an opening larger than the diameter of the ball. Manure particles are irregular in shape, so we define the characteristic dimension of the particle as the size of the screen opening that will retain the particle. Consider a strand of hair. The length of the hair will most often determine if the hair passes through a screen; therefore, the characteristic dimension for a hair is its length. Particle shape, particle density, and fluid viscosity determine how a particle will settle in a suspension.
Settleable solids
Suspended solids are arbitrarily determined by whether the characteristic dimension of the particles are larger than 1.5 microns. This also happens to be a good estimate of the smallest sized particle that can be removed by settling. A standardized test has been devised to determine the volume of solids that settle out of liquid. A volume of slurry is placed in a container called an Imhoff cone, and allowed to settle for one hour. Results are given as volume of settled sludge divided by the original volume of the liquid. For instance, 100ml/l means that for 1 liter of slurry, we can expect 100ml of cake to settled out of the slurry after one hour. Results given on a volume basis do not give the TS concentration of the sludge, only the amount of sludge that will settle out of the waste stream.
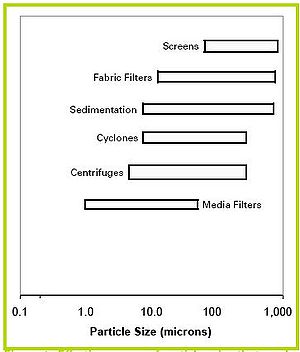
Separation methods
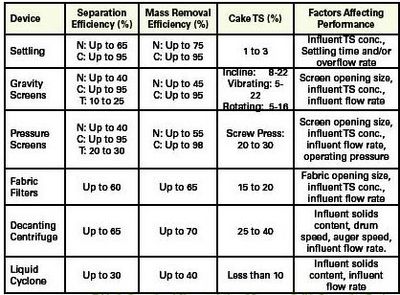
Throughout the years, environmental engineers have devised a number of techniques and devices to separate solids from liquids in wastewater. Figure 4 gives the approximate particle size range that can be removed by each technique. The two techniques most commonly used in swine manure handling are settling (sometimes called sedimentation) and screening. Fabric filters and centrifuges for swine waste are in development, and some research has been performed on flotation devices and media filters. Operational characteristics of common solids separation devices are given in Table 1.
Settling
The settling rate of particles is affected by size, shape, and density of the particle, and the density and viscosity of the liquid they are passing through. If a particle sufficiently large and dense, it will settle out of suspension as a discrete particle—as a rock drops through a column of water. As a particle settles, it accelerates until the frictional drag on its surface equals the weight of the particle in the suspending fluid. Once friction and gravity are equal, the particle travels downward at a constant velocity called its terminal velocity. Particles free-falling at their terminal velocity are said to be undergoing Type I or Type II settling. The distinction between type I and type II settling is that type I takes place when the particle is a discrete piece of material such as a grain of sand. Type II settling takes place when the particle is a mass of smaller particles stuck together.
If there are many particles falling at the same time, the relative velocity between the particle and the fluid passing over it increases since the space between particles is smaller—if the space is smaller, and the amount of fluid remains the same, the speed at which the fluid flows must increase. The individual particles slow down as the relative velocity of fluid acting against them increases. If the concentration is great enough, the fluid velocity between particles causes the solids to settle as a group with uniform concentration. Settling solids appear as a cloud with a distinct boundary between the top of the cloud and the clear liquid above it. Particles settling as a cloud is called Hindered or Zone settling. The speed at which the border between clear liquid and the cloud passes through a column is the settling velocity of the slurry.
Settled particles form a sludge layer at the bottom of a column. When the free-falling or hinder settling particle reaches the top of the sludge layer, it decelerates as the space between particles becomes smaller, and the velocity of liquid increases. The pressing of particles together squeezes liquid between them. Concentration of solids increases the deeper into the sludge layer. Settling that takes place as the concentration of particles in suspension increases is called Compression.
Factors affecting settling of manure
As you can see from Table 1, mass removal efficiency of settling can be quite high. You might be disappointed in your attempt to settle solids, though, because efficient settling depends on numerous factors.
Influent solids content
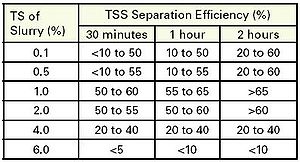
Table 2 is a compilation of studies showing how separation efficiency and settling velocity are affected by influent TS concentration. As you can see from Table 2, settling is most efficient and predictable when initial TS concentration is between 1 to 2%. At less than 1% TS, interaction between particles tends to keep them in suspension. Greater than 2% TS, liquids become too thick or viscous for particles to settle. The phenomena shown in Table 2 can work for or against us at different parts of the manure handling system. We want solids to be suspended as they are removed from a building; therefore, flushing and pit recharge systems are designed to operate at less than 1%TS. Once slurry reaches a settling tank, though, we would like TS to be greater than 1% for efficient settling.
Overflow rate
The data in Table 2 were collected from experiments in which slurries settled in perfectly still columns. Perfectly still conditions are hard to achieve in a settling basin, especially if influent is constantly entering the basin. For solids to settle in a continuously flowing system—liquor must be drawn off the top of basin at the same rate influent enters, and cloud of settling solids must stay clear of the draw-off area. This leads to a design calculation called the overflow rate:
(22) Overflow rate = influent flow rate/ surface area of settling tank
If influent flow has dimensions m3/min and the surface area has dimension m2, overflow rate has the dimensions m/min, the same dimension as settling velocity. If overflow rate is less than settling velocity, then separation efficiency of the tank should be equal to the maximum achievable efficiency for a given influent TS concentration.
Compression
You also have to remove cake from the tank at a rate equal to or less than the settling velocity for cake to accumulate. The volume of cake generated in settling basins is generally too great to allow cake sufficient time to compress unless flow is directed to more than one tank. Total solids during zone settling will be at some concentration greater than the influent TS, but rarely greater than 3 or 4% TS. With long compression times, you can hope to increase cake TS to about 6% TS.
Cake volume. With high mass removal efficiency and low cake TS, it is easy to see that large volumes of cake are produced during manure settling. If a system has 1.0 % TS influent, 1.25% TS cake, 0.35% TS liquor, and 65% separation efficiency, volume of cake and liquor generated by the system would be roughly equal. This may make for trouble storing all the thin cake, but mass removal efficiency is great—83%.
Screening
Screening devices come in two general varieties, those that use gravity to separate solids, and those that pressurize influent to force liquids through a screen.
Gravity screens

As you might expect, separation efficiency is largely dictated by screen opening size. Figure 5 is a plot of separation efficiency versus screen opening size taken from a large number of studies using many types of gravity screens to separate untreated swine manure. Settling efficiency falls below a maximum probable line that is a function of screen size. Comparing Figure 5 to Figure 3 shows that the maximum probable line roughly coincides with percent solids retained for the three diets.
Maximum separation efficiency can only be achieved with high solids influent and long residence times on the screen. Both of these conditions also tend to increase clogging or blinding of the screen, so most of the results shown in Figure 5 fall short of what can be theoretically achieved. In practice, screens used to separate swine solids have screen sizes between 500 and 1000 microns, hence the potential separation efficiency of gravity screens is less than 50%. And under farm conditions, the actual separation efficiency is more likely to be less than 30%. Mass removal efficiency depends on concentration of the cake.
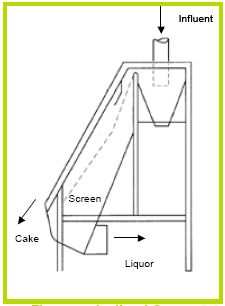
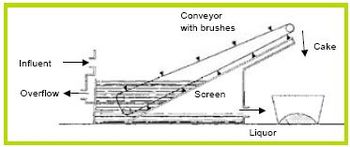

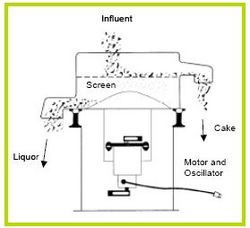
Gravity screening devices come in a number of different varieties, such as, Inclined Screens (Figures 6 and 7), Rotary Screens (Figure 8), and Vibrating Screens (Figure 9). Separation method of all theses devices is the same—a screen blocks particles from passing. Differences are the innovative ways the manufacturer uses to increase efficiency for a given screen size by reducing clogging and increasing throughput.
TS concentration of cake from gravity screens can be anywhere from 5 to 20%. The main factor affecting concentration is retention time on the screen. Gravity screens can only remove gravitational liquids from the solids, and total removal of gravity water is only possible with an infinite retention time. Cake from gravity screens will usually ooze some additional liquid when stacked.
Pressure screens
The difference between a gravity screen and a pressure screen is that a pressure screen uses increased influent pressure to force water through the screen. Separation efficiency is slightly better than gravity screens (Table 1), because pressure screens generally have smaller openings than their gravity counterparts. The main advantage of a pressure screen is that solids concentration of cake can be increased over that of a gravity screen. Pressure screens remove some hygroscopic, as well as gravity liquids, so cake produced by a properly operated pressure screen device should not ooze when stacked.

The most common form of pressure screen used in agriculture is the Screw Press (Figure 10). Influent is pumped into the device and moved though a cylindrical screen with a helical screw. Pressure is maintained by restricting flow through a small hole (orifice) at the end of the screen. Cake TS is dependent on pressure. Increase pressure and more liquid is squeezed out of the cake, thus increasing cake solids concentration. Cake TS concentration does not depend upon influent TS to a large extent.

Mass removal efficiency and liquor flow rate of screw presses are affected by influent TS. Figure 11 shows that mass removal efficiency increases and liquor flow rate decreases with increased influent solids for a screw press separating dairy manure. Analysis of the data used to plot Figure 11 shows that the separation efficiency also increases with influent TS, and reaches a plateau of 40% with 3% TS influent. The increase in separation efficiency may because higher solids manure contains larger, conglomerated particles than dilute manure.
Increasing mass removal rate at higher solids concentration comes at the expense of liquor flow rate. Based on the data used to plot Figure 11, if cake TS is relatively constant at 20%, influent flow rate is roughly 1.25 times the liquor flow rate. Screw presses can be very efficient, and deliver a good quality cake, but the efficiency requires a thick influent and flow rates considerably lower than the maximum rated for the equipment.
Filtering with fabric

The treatment method of fabric filters is very much like screens— particles cannot pass through holes in the fabric. Fabric openings are much smaller than those of screens, so separation and mass removal efficiency is high (Table 1). Most fabric filters use some form of pressure to squeeze liquor from cake; therefore, cake is generally in a semi-solid or solid form. The pressure may come in the form of an external press, or cake may be squeezed between two belts of fabric. Figure 12 shows a Belt Press Filter, in which a continuous belt of filter fabric moves material through the system. Press rollers squeeze liquor from the cake, and a rotary brush removes cake that might stick to the belt.
Vacuum drum filters are similar to rotary screens shown in Figure 8, only the screen is replaced with filter fabric. A vacuum is induced on the inside of the drum to draw liquor into it by negative pressure. Solids stick to the filter fabric and are removed with a metal edge as the drum rotates.
Filtering with media
Filters using media, such as sand or synthetic material, can achieve a high rate of separation efficiency and remove smaller particles than other techniques (Figure 4). Thin media filters, such as sand beds, function as dryers as well as separators. Deeper media filters usually perform biological treatment in addition to separation.
Centrifugal force
Two types of devices use centrifugal force to separate solids from liquids: centrifuges and hydrocyclones. Both rotate the solid/liquid mixture and force particles to move to the outside of the rotating motion. In effect, an artificial gravity is created, and particles move by the force of gravity as they do during settling. Since centrifugal forces can be greater than the earth gravitation field, centrifuge devices can achieve separation efficiencies equal to or better than settling while producing higher TS cake.
Decanting centrifuge
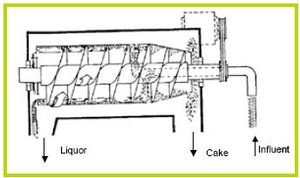
Decanting centrifuges consist of a horizontal or vertical cylinder which is continuously turned at high velocities (Figure 13). Centrifugal forces press solids onto the inside wall of the cylinder. An auger, which turns slightly faster than the cylinder, moves the cake back to the conic part of the unit where it is discharged. Decanting centrifuges can attain high separation efficiency producing semi-solid cake. They require influent TS concentrations in the 5-8 % TS range, and are considerably less efficient when operated with more dilute influent.
Hydrocyclones

Hydrocyclones are cone-shaped separators that have no moving parts (Figure 14). Slurry is pumped at an angle near the top of the cone. A vortex motion is thus created in the liquid and the strong swirling motion increases the gravity settling of solids to the bottom of the cone. Liquid leaves the cone from the top. Separation efficiency of hydrocyclones is not as great as decanting centrifuges, but they are useful in separating dense particles such as precipitating crystals from mixtures.
Flotation
Floatation devices also use gravity, but have the opposite effect of settling basins or centrifuges. The idea of flotation is to separate particles lighter than the liquid with which they are mixed. A common flotation system, called Dissolved Air Flotation or DAF, introduces air under pressure into a conical tank. The fluid pressure inside the tank is considerably lower than the vapor pressure of the dissolved air; therefore, bubbles form and rise to the top of the tank, carrying light, and suspended particles with them. DAF has not been used extensively in agriculture; therefore, limited data is available to judge its effectiveness separating swine manure slurries.
An Aerated Grit Chamber combines both flotation and sedimentation mechanisms. Air rises through the chamber carrying light particles to the surface. At the same time, dense particles settle to the bottom because the rising bubbles reduce their buoyancy in the liquid. Aerated grit chambers have been used successfully to separate sand bedding from dairy waste slurries.
Chemical enhancement of solid liquid separation
As eluded to in Table 1, separation and mass removal efficiency of solids separators can be greatly improved using chemicals to enhance the separation process. Chemical agents increase separation efficiency by enhancing the processes of coagulation and flocculation.
Coagulation
When manure particles are dispersed in water, they carry a small negative electrical charge. Opposite charges attract and like charges repel. This negative charge is strong enough to keep small particles from coming close together. Adding positively charge particles or chemicals to a mixture collapses the negative charges and allows the particles to move closer together. The process of collapsing the negative charge on particles is called coagulation, and chemicals that induce coagulation are called coagulants. Coagulants used with manure come in two varieties. The first variety are salts of positively charged metals cations. The most common metallic salt coagulants are Alum (Aluminum sulfate, Al(SO4)3), Ferric chloride (FeCl3), and lime (CaO). A second group of coagulants are organic polymers that carry positive charges, or cationic polymers. The most common polymer used to coagulate manure mixtures is polyacrylamide or PAM.
Coagulants should be added to slurries with vigorous mixing to completely mix the positive charges amongst the suspended particles. Cationic coagulants are also most effective at pH higher than 7. Although coagulants increase the amount of solids settled, they also increase the time needed for a cloud of solids to settle.
Metallic salts are very effective coagulants, and given the proper dosing rate, they will virtually remove phosphorus from manure slurries through sedimentation. Metallic salt coagulants form large volumes of gelatinous cake that cannot be used in combination with more rigorous separation techniques such as screening, belt presses, or centrifuges. The gooey gelatin sticks to fabrics, and clogs screens. Some metallic salts, such as Lime and Ferric chloride, are caustic materials, and should be handled with care to avoid harming people or equipment. Some metallic salts are also toxic to plants and animals, so their use must be adjusted to avoid problems during land application.
Cationic polymers are weak coagulants, and are relatively ineffective at removing phosphorus from manure. Only cationic polymers have been shown to be effective with manure mixtures, and polymers with medium charge density (30-50 mole %) work best; although high charge density polymers (greater than 50 mole %) have also been shown to work effectively on very dilute slurries. On the positive side, cationic polymers are not toxic to plants and animals. Polymers are also flocculants, which we will see in the next subsection create strong solids able to withstand all separation techniques.
Flocculation
Once coagulation occurs, more and more particles begin to stick together are start to settle out of suspension. As the particles settle as a cloud, they bump into other particles, stick together and form even larger particles. These large particles are called flocs. The process of forming flocs is called flocculation, and chemicals used to enhance flocculation are called flocculants. The most common flocculants used with manure mixtures are various forms of polyacrylamide or PAM. Polymers are long chains of organic chemicals. The strands of polymers help bind soft flocs of manure particles together. Think of particles being caught in a spider’s web or fish net.
The binding of particles not only makes flocs larger, it also makes the flocs stronger. Using flocculants aids in the removal of solids using screens, fabric filters, and centrifuges. Solids formed using a combination of coagulant and flocculant are more compact that those formed using coagulant alone. Flocculation requires gentle mixing action: vigorous enough to bring the flocculant in contact with particles, but not so vigorous to break up flocs.
Effectiveness of chemical treatments
Metallic salts
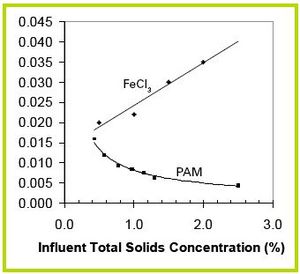
Addition of metallic salt coagulants has been shown to raise TSS separation efficiency of settling from 60% without chemicals to over 90%. Phosphorus separation efficiency with metallic salts is 75-90%. The most effective salts are Alum and Ferric Chloride.
The optimum dosing rate for alum in a 1% TS manure slurry has been shown to be 1500mg/l or 0.15 part alum per part influent TS. Although metallic salts increase the separation efficiency of settling, they also decrease settling velocity, thus increasing the time required for slurry to settle. Optimum dosing rate of Ferric chloride versus influent TS concentration based on settling velocity is shown in Figure 15. All the dosing rates shown in Figure 15 increased separation efficiency over 90%. As you can see from Figure 15, more ferric chloride is required as to settle suspended solids as influent TS increases; therefore, metallic salt coagulants are most efficient when used with relatively dilute slurries.
Polymer
Addition of polymer coagulants-flocculants has been shown to raise TSS separation efficiency of screens from less than 20% to over 90%. Polymer addition increases removal of organic phosphorus, but does not affect inorganic dissolved phosphorus. This polymer will only slightly increase phosphorus separation efficiency of slurries made of manure excreted by pigs fed traditional diets, since the inorganic phosphorus fed to the pigs remains dissolved in the manure slurry.
Figure 15 shows the optimum dosing rate of medium charged cationic polymer per influent total solids based on separation efficiency. The dosing rates shown in this figure resulted in separation efficiencies from 87-96% using a 1,000 micron screen following settling. Unlike Ferric chloride, efficiency of polymer increases as influent TS increases. This makes sense because of the natural tendency of particles to flocculate as the concentration of solids in the slurry increases. Because there are more particles colliding naturally, there is less need for polymers to bring the particles together.
Because of the increase in efficiency at higher solids concentrations, producers are often encouraged to increase the TS of manure removed from the buildings when using polymers for settling. This has its limitations. At some point, the slurry will be too thick to flow out of the building and down a pipe. As with all recommendations, this must be taken with moderation.
Combination of metallic cations and polymer. Using a combination of metallic cations and polymers allows an operator to have the best of all possible systems: near complete removal of phosphorus, over 90% solids removal with screens, efficient use of chemicals with dilute slurries, and reduced toxic effect of the metallic salts. Combining metallic salts and polymer may also reduce costs since the price of metallic cations is considerably less than polymer (April 15, 2004 prices: Ferric chloride is $0.36/kg, PAM is $4.60/ kg). Research has shown that polymer use in screened dairy manure could be reduced to as little as one third of the optimum dosing rate when used in combination with Ferric chloride at its optimum dosing rate. Separation efficiency may suffer a little: 90% of TSS versus 96% when polymer is used alone.
Summary
Solids separators separate flow of solids-laden influent into two waste streams: low-solids liquor and high-solids cake. The extent to which the solids separator is able to decrease solids content of the liquor is measured by separation efficiency. The extent to which the device can transfer solids to cake is measured by mass removal efficiency. The equation, QiCi = QcCc + QlCl, governs the flow into and out of a solids separator. It is possible to have very high mass removal efficiency, but achieve little benefit to the manure handling system if cake solids concentration is low, resulting in volumes of cake as great as treated liquor. Solids separators use settling, screening, filtering, centrifugal force, and flotation to remove solids from slurry. Each type of separator has benefits and drawbacks. Selection of components should depend on what will benefit operation of the system, not an arbitrary standard such as mass removal efficiency. Chemical coagulants and flocculants can greatly improve the performance of solids separator but at added cost. The most effective system is likely to be one that combines the benefits of multiple subsystems. As with all treatment scenarios, solids separation increases the overall cost of handling manure. The costs and benefits of solids separation must be balanced against the overall cost and potential income of production to make sound business decisions.
Literature Cited
1. USDA-NRCS, 1992. National Engineering Handbook Part 651, Agricultural Waste Management Field Handbook. United States Department of Agriculture – Natural Resources Conservation Service: Washington, DC.
2. Jett SC, Ross IJ, Hamilton HE, Hays VW. Size distribution and nutritional value of swine manure separates. Transactions of ASAE 1974:965.
3. Nalco Chemical Company. The Nalco Water Handbook, 2nd Edition, Frank N. Kemmer, ed. McGraw Hill: NY. 1988.
4. Moore JA, Hegg RO, Scholz DC, Strauman E. Settling solids in animal waste slurries. Transactions of ASAE. 1975:694.
5. Cullum RF. Sedimentation phenomena in swine waste slurries: model development. Transactions of ASAE. 1988;31(6):1760.
6. Ndegwa PM, Zhu J, Luo A. Effect of solids levels and chemical additives on removal of solids and phosphorus in swine manure. Journal of Environmental Engineering. 2001;127(12):1111.
7. Zhang RH, Westerman PW. Solid-liquid separation of animal manure for odor control and nutrient management. Applied Engineering in Agriculture. 1997;13(3):385.
8. Moller HB, Lund I, Sommer SG. Solid-liquid separation of livestock slurry: efficiency and cost. Bioresource Technology 2000;74:223.
9. Hegg RO, Larson RE, Moore JA. Mechanical liquid-solid separation in beef, dairy and swine waste slurries. Transactions of ASAE. 1981:159.
10. Burns RT, Moody LB. Vincent KP-6L solids separator performance test results using the University of Tennessee testing protocol, AWM-01-02. University of Tennessee Agricultural and Biological Engineering Department: Knoxville, TN. 2001.
11. Glerum JC, Klomp G, Poelma HR. The separation of solid and liquid parts of pig slurry. In: Waste Management and Pollution Abatement, Proceedings of the International Symposium of Livestock Wastes; ASAE: St Joseph, MI. 1971. p. 345-347.
12. Shutt JW, White RW, Taiganides EP, Mote CR. Evaluation of solids separation devices. In: Managing Livestock Wastes, Proceedings of the 3rd International Symposium on Livestock Wastes; ASAE: St Joseph, MI. 1975. p. 463-467.
13. Zhang RH, Lei F. Chemical treatment of animal manure for solid-liquid separation. Transactions of ASAE. 1998;41(4):1103.
14. Vanotti MB, Rashash DM, Hunt PG. Solid-liquid separation of flushed swine manure with PAM: effect of wastewater strength. Transactions of ASAE. 2002;45(6):1959.
Table 1. Operational Characteristics of Common Solids Separation Devices. N = Naturally, C = Chemically, T = Typically
Table 2. Suspended solids separation efficiency of homogenous columns of slurry at various times after slurry was allowed to settle [4-6].
Figure 1. A solids separator is a treatment component of a manure handling system that divides an influent waste stream into low solids liquor and high solids cake.
Figure 2. Relationship between solids concentration and manure consistency for swine, poultry, beef, and dairy manure [1]
Figure 3. Particle size distribution for swine manure slurries resulting from three types of diets [2]
Figure 4. Effective ranges of particles size that can be removed by common separation methods [3].
Figure 5. Relationship between screen openings and separation efficiency for gravity screens [7].
Figure 6. Inclined Screen [1].
Figure 7. Inclined Screen [8].
Figure 8. Rotary Screen [9].
Figure 9. Vibrating Screen [1].
Figure 10. Screw press [8].
Figure 11. The effect of influent TS on mass removal efficiency and liquor flow rate for a Vincent KP-6L screw press separating dairy manure [10]
Figure 12. Belt Press Filter [1]
Figure 13. Horizontal Decanting Centrifuge [11].
Figure 14. Hydrocyclone [12].
Figure 15. Optimum dosing rates of Ferric Chloride and Medium Charge Density Cationic PAM based on influent TS concentration [13,14].
